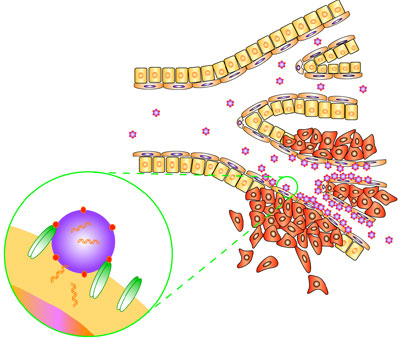
Nanocarriers have several advantages over conventional chemotherapy. They can:
- Protect drugs from being degraded in the body before they reach their target
- Enhance the absorption of drugs into tumors and into the cancerous cells themselves
- Allow for better control over the timing and distribution of drugs to the tissue, making it easier for oncologists to assess how well they work and
- Prevent drugs from interacting with normal cells, thus avoiding side effects
Passive targeting
There are now several nanocarrier-based drugs on the market that rely on passive
targeting through a process known as "enhanced permeability and retention."
Because of their size and surface properties, certain nanoparticles can escape
through blood vessel walls into tissues.
In addition, tumors tend to have leaky blood vessels and defective lymphatic
drainage, causing nanoparticles to accumulate in them: this concentrates the
attached cytotoxic drug where it's needed, protecting healthy tissue and greatly
reducing adverse side effects.
Active targeting
Nanoparticles are being developed that will actively direct drugs to cancerous cells,
based on the molecules that they express on their cell surface. Molecules that bind to
particular cellular receptors can be attached to a nanoparticle so that they actively
target cells expressing the receptor.
Active targeting can even be used to bring drugs into the cancerous cell, by inducing
the cell to absorb the nanocarrier. Active targeting can be combined with passive
targeting to further reduce the interaction of carried drugs with healthy tissue
achieving greater tumor reduction using lower doses of the drug.
Destruction from within
Moving away from conventional chemotherapeutic agents that activate normal
molecular mechanisms to induce cell death, researchers are exploring ways to
physically destroy cancerous cells from within.
One such technology has used 'nanoshells' in the laboratory to thermally destroy
tumors from the inside. A nanoshell consists of beads that are about three
millionths of an inch wide, with an outer metal wall and an inner silicon core.
Nanoshells can be designed to absorb light of different frequencies, generating heat
(hyperthermia).
| |
|
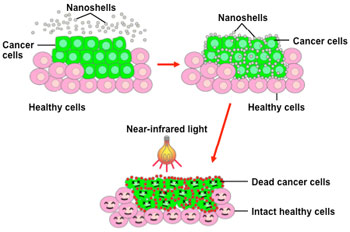 |
- Once the cancer cells take up
the nanoshells (via active
targeting), scientists apply nearinfrared
light that is absorbed by
the nanoshells.
- This creates intense heat inside
the tumor that selectively kills
tumor cells without disturbing
neighboring healthy cells.
|
| |
|
| Image courtesy of the National Cancer Institute |
Similarly, targeted magnetic nanoparticles are in development that will not only be
visible when using Magnetic Resonance Imaging (MRI), but also destroy cells by using
hyperthermia.
Examples of approved nanotechnologies for cancer
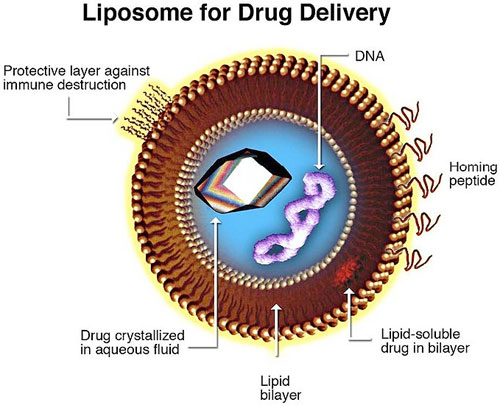
Liposomes, which are first generation nanoscale devices, are being used as drug
delivery vehicles in several products. For example, liposomal amphotericin B is used
to treat fungal infections often associated with aggressive anticancer treatment.
| |
|
| |
| Image courtesy of Dr. Kosi Gramatikoff |
The medication Doxil also uses liposomes: liposomal doxorubicin is used to treat
certain myeloma and ovarian cancers. Doxil takes the active chemotherapy drug
doxorubicin and places it into a liposome and another layer of hair-like strands
made from a rubber called methoxypolyethylene glycol.
This coating allows Doxil to evade detection and destruction by the immune system
thus increasing the time the drug is in the body. At least 90 percent of the drug stays
inside the liposome while in the blood. As a result, Doxil has more time to reach the
tumor tissue where the medication slowly leaks out.