The Promise of Tumor Markers - page 2
What is the availability of tumor markers?
The use of some tumor markers is considered standard care in the cancer care community, while others are used less frequently.
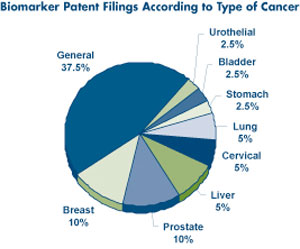 |
This image depicts patent filings for biomarker research by cancer type. |
Image courtesy of Insight Pharma Reports |
|
Health insurance companies may or may not provide coverage for tumor marker tests. Ask your oncologist about the use of tumor markers for your diagnosis, and how markers are combined with other strategies for diagnosis and monitoring of cancer.
What is the future for tumor markers?
Research and clinical experience have revealed that testing a single protein or other substance in people may not be the most reliable marker of disease. Other research approaches may determine better tumor markers.
There are not only many elements but also many technologies that need to be verified and standardized. Many researchers are working to move this work into the clinic as soon as possible.