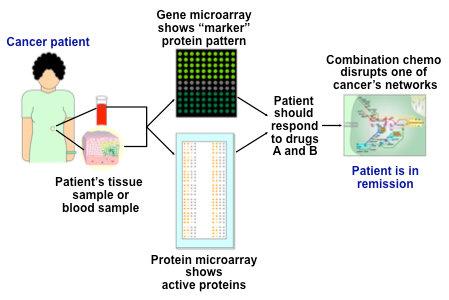
Those molecular diagnostics that are currently available remain relatively limited. In addition, many may be expensive, perhaps with the exception of the Pap smear, which has long been effective in detecting early-stage cervical cancer.
However, some molecular diagnostic tests are currently used as or are becoming standard care. See examples given in earlier in this section under “biomarker tests.” In addition, some treatments developed through research on molecular diagnostics, have been approved by the FDA for use in specific types of cancer.
Molecular diagnostics may also be available through participation in clinical trials (research studies in people). Health insurance companies may or may not provide coverage for specific molecular diagnostics.
Ask your oncologist about molecular diagnostics for your diagnosis and whether an appropriate targeted agent has been approved or is under study for your specific cancer type.