Despite the rising costs associated with new drugs, industry is committed to providing a more targeted approach to medical treatment.6 It’s suggested that more personalized prescribing may lead to impressive financial benefits, as an estimated 40% to 70% of patients currently may not respond to available treatments for conditions such as depression, asthma, diabetes, arthritis, and cancer.7
However, many new therapies will be more expensive to develop, because research funds may not be available for cancer subtypes that affect small numbers of people, thus providing less opportunity for profit.
Food & Drug Administration (FDA) regulations have specified that companion diagnostics used to direct drug therapy choice should:
- Be considered Class III medical devices
- Follow the Premarket Approval (PMA) process, which is the strictest level of FDA device review and one that typically requires appropriately designed and conducted clinical trials.8
Recently, the FDA released a draft guidance document to clarify its position on the use of non-FDA-approved molecular tests in labs reporting clinical results.
The development and clinical validation of molecular diagnostic clinical tests deserves the same level of thoroughness as required for drug development. In addition, they must be offered at a realistic cost that reflects both their clinical value and the costs associated with their development.9
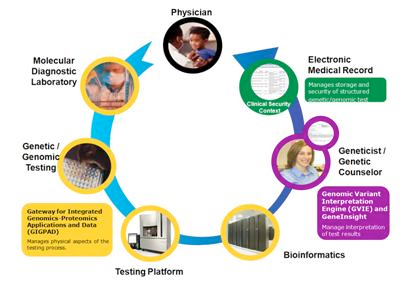 |
| Image courtesy of Health & Human Services.gov news |



