Clinical Trial Phases for Cytotoxic Therapies - page 2
Phase III Clinical Trials
If a certain percentage of the patients benefit from the treatment in a Phase II trial and the side effects aren't too severe, it is considered to be active enough against that type of cancer to warrant it moving on to a Phase III clinical trial.
Goal of phase III studies: Is it more effective than standard of care?
Phase III clinical trials compare the safety and effectiveness of the new treatment against the current standard treatment (or standard of care).
The choice of primary endpoint is a critical element in the design of these trials. Typically some element of survival is evaluated (primary endpoints discussed previously). Many studies have shown little correlation between response rates and survival; consequently, response rates are not used as primary endpoints for most Phase III trials.
How many people are enrolled? Large numbers around the country
Risk vs. benefit? This phase trial has the least risk of side effects and may show benefit for patients although patients may be randomized into the standard of care arm and not receive the new agent/technique.
How long will the study take? Two - five years or more
Summary: In order to establish that one therapy is better than another, clinical researchers must determine in advance what degree of difference between the new and the standard treatment will be considered clinically important. The most important characteristic of Phase III studies is randomization, a study design feature that provides an unbiased assignment of patients to one or the other treatment. Statistical analyses of such trials that evaluate the likelihood that the difference in outcomes observed is not due to chance alone are very important.
Phase IV Clinical Trials
Once a drug is approved for use on the basis of the data derived from earlier-phase trials, its safety and efficacy during routine practice may be evaluated in a Phase IV trial (post-marketing trial). Sometimes these studies are mandated as part of the approval process. The results of such studies rarely lead to withdrawal of the treatment from the market but it can happen.
Less common or long-term side effects are sometimes detected during these trials, since very large numbers of patients are typically exposed to the new treatment/intervention.
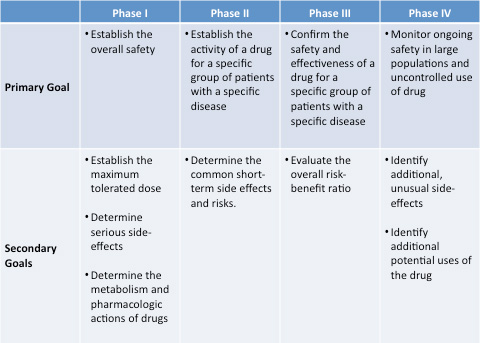
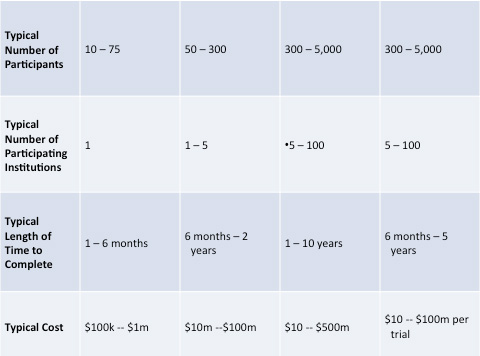
Summary of all phase in chart below:
|